八氟紫質的合成
Synthesis of Octafluoroporphyrin
八氟紫質的合成
Angew. Chem., Int. Ed., 2016, 55, 5035-5039
DOI: 10.1002/anie.201511702
Chiranjeevulu Kashi, Chu-Chun Wu, Chi-Lun Mai, Chen-Yu Yeh,* Chi K. Chang*
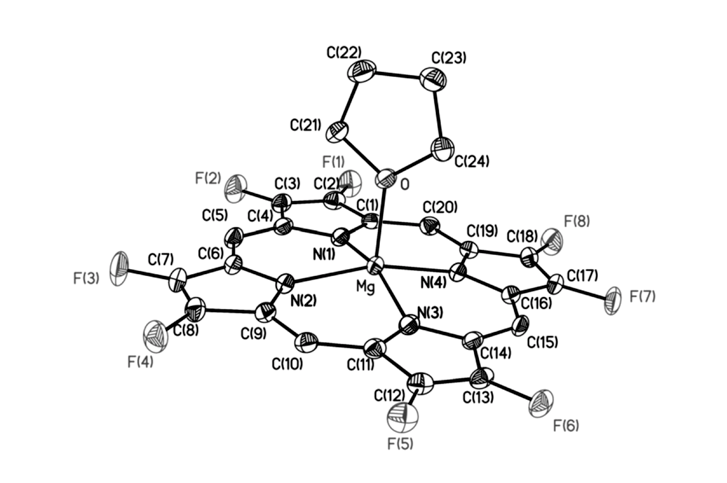
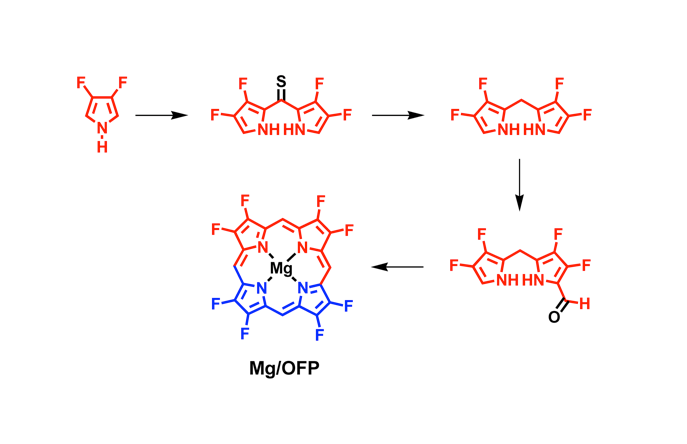
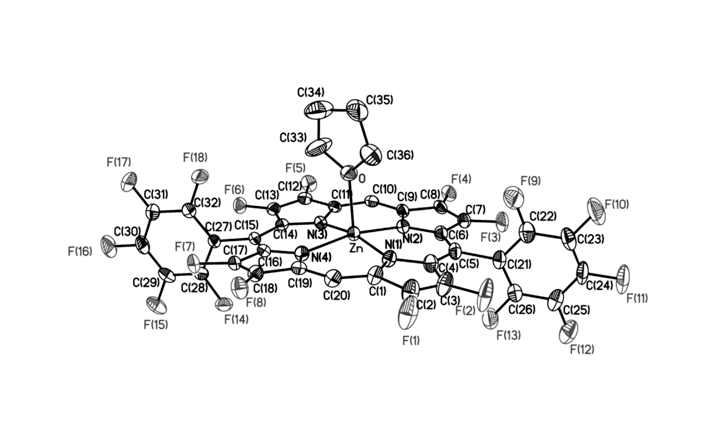
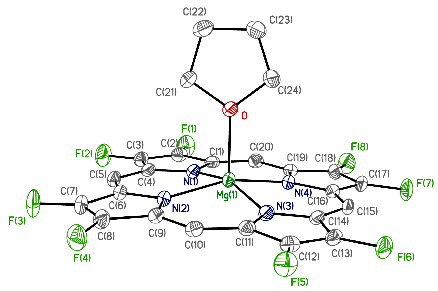
Fluorinated porphyrinoids are interesting for many reasons. Apart from the fact that fluoroorganic molecules are of increasing importance as bioactives in life science, the electron-deficient nature of fluorinated porphyrinoids renders them ideal systems for modeling biomimetic mono-oxygenations, such as Fe/F20TPP and Mn/F20TPP for modeling cytochrome P450. With the current state of the art in porphyrin syntheses, most porphyrins, biological or otherwise, have either already been made or may be assembled without great difficulty. Yet, 2,3,7,8,12,13,17,18-octafluoroporphyrin (OFP) is one seemingly simple and fundamental compound that has eluded all synthetic attempts for over two decades. Fortunately, it is achieved in this study by condensation of two molecules of tetrafluoro-dipyrrylmethane-2-carboxaldehyde in the presence of magnesium(II) salts. The fluorinated dipyrrylmethane also gives 5,15-bis(pentafluorophenyl)-OFP (F18P) with a reasonable yield. Both Mg/OFP and Zn/F18P in the solid-state reveal an essentially flat structure. The fluoro groups impart as much as a 0.5 V anodic shift for porphyrin ring oxidation/reduction, as well as hypsochromic shifts in the UV-vis spectra.
幾十年來,氟化紫質之所以讓研究人員如此著迷,其中有許多原因。除了含氟有機分子在生命科學中作為生物活性劑的重要性越來越重要之外,氟化紫質的缺電子性質使其成為建構仿生單氧化酶的理想系統,如用於模擬細胞色素P450的Fe/F20TPP和Mn/F20TPP。時至今日,以現有技術,要合成大多數生物紫質或其它類紫質已不是件困難的事情。然而在過去超過二十年的時間,2,3,7,8,12,13,17,18-八氟紫質(OFP)這種結構上極為簡單且基本的化合物,卻難倒了全世界的合成化學家。幸運的是,在本研究中,我們透過在鎂(II)鹽存在下的兩個四氟-二苯乙烯基甲烷-2-甲醛分子之縮合反應來實現鎂取代八氟紫質、代號Mg/OFP之合成。氟化二苯乙烯基甲烷可以合理的產率得到5,15-雙五氟苯基八氟紫質,代號為F18P。藉由X光晶體繞射之鑑定,我們確定了Mg/OFP和Zn/F18P都顯示出基本平坦的結構。透過電化學研究得之,OFP結構上的多個氟取代基團為紫質之氧化及還原電位帶來了多達0.5V的氧化性位移,而紫外可見光譜之研究也指出,多個氟取代基可造成紫質紫外-可見光吸收的藍位移。
Attachment



